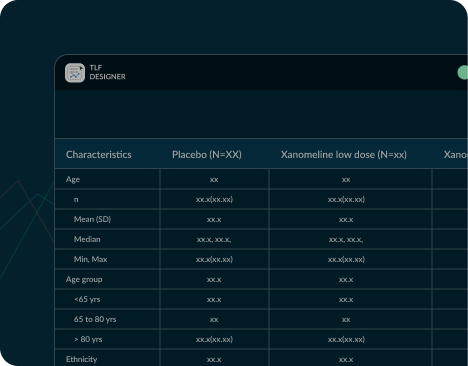
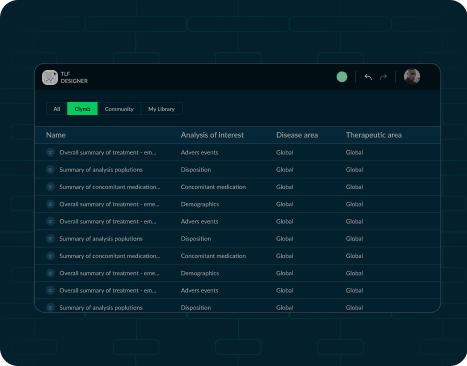
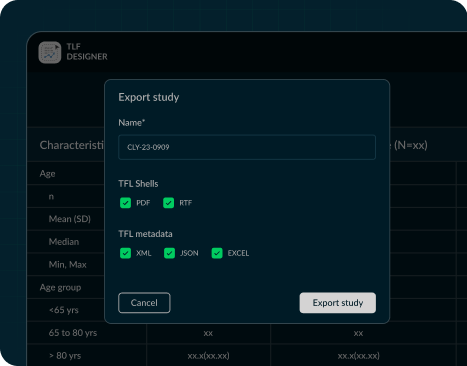
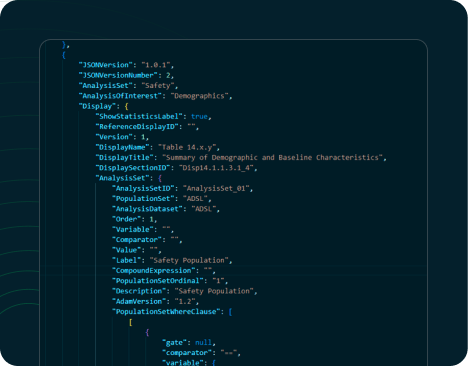
TFL Designer, or Tables, Figures, and Listings Designer,
is a specialized tool developed by
Clymb Clinical
to streamline the creation of TFLs in clinical trial data analysis. It digitizes and
automates the design
of these essential components, offering machine-readable analysis results metadata. The
benefits of using
TFL Designer include improved efficiency in TFL creation, customization flexibility,
alignment with CDISC
Analysis Results Standards, and support for downstream automation processes including
generation of
Analysis Results Data and TFL codes/outputs. This robust solution streamlines data analysis
workflows
(keeping end in mind - going from study objectives to data collection i.e. TFL → ADaM → SDTM
→ CRF),
leading to time savings, enhanced data quality, and more effective decision-making.
In addition to these advantages, we are actively working
on a community free version. This
initiative will
empower the industry with broader access to TFL Designer, fostering collaboration and
knowledge sharing
among stakeholders. Users of the community version will benefit from a growing library of
templates, use
cases, and standards, accelerating TFL design and automation. Furthermore, the
incorporation of FDA
templates, based on recent FDA Safety Tables and Figures - Integrated Guide (STF-IG), in the
community
version enhances its utility and aligns it with the latest regulatory requirements. By
offering a
community version, we aim to contribute to a more efficient and standardized approach to
clinical trial
data analysis across the industry.